SALUS
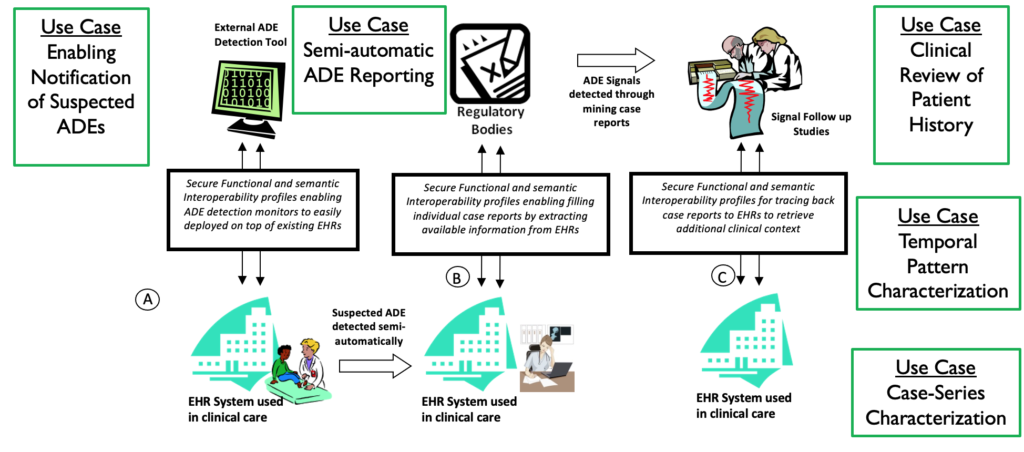
SALUS is the acronym for the successfull FP7 project: Scalable, Standard based Interoperability Framework for Sustainable Proactive Post Market Safety Studies. As illustrated in the figure below, SALUS worked on the following:
- Functional interoperability to enable the exchange of electronic health records. For this, a new IHE profile was developed by the SALUS project and I was one of the authors of this profile.
- Semantic interoperability to align the data elements of the exchanged electronic health records, such as coded fields using health data terminologies. We developed several ontology systems to represent health datasets and their mappings including the terminology systems. The mappings were performed through the ontology system utilizing RDF, OWL and their different serializations utilizing the Apache Jena framework and EYE reasoning engine.
- A configurable security and privacy framework to eliminate the identiying information during the processes electronic health records and application of the secondary use analytics algorithms.
- Implementation of temporal patterna association and use-case characterization algorithms to detect potential signals on the integrated electronic health records.
- A semi-automatic Adverse Drug Event reporting tool so that the adverse events can be easily reported to the autorhirities by the medical professionals.
I acted as the technical manager of the whole international project covering 10 different organizations around Europe and spanning several work packages. It was a great experience for me to collaborate with professionals from different domains and countries and bring an harmony to achieve the successful results of the SALUS project. I worked hard, travelled a lot and authored many papers and talks during the project. Thanks to the SALUS project that it led to my PhD thesis where I designed and developed a new data interoperability approach through metadata registries and evaluated it with the help of SALUS partners. This paper describes the federated data interoperability approach of my PhD these. Semantic MDR was a side-product of my PhD study as well.
Description
Framework Programme 7 (FP7)
2012 - 2015
Scalable, Standard based Interoperability Framework for Sustainable Proactive Post Market Safety Studies
